Copper-catalyzed cyclization reaction: synthesis of trifluoromethylated indolinyl ketones†
Abstract
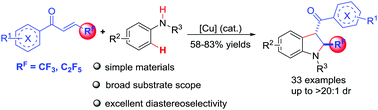
A novel, simple, effective and rapid synthetic method to construct the C-2 trifluoromethylated indolinyl ketones via a copper-catalyzed cyclization reaction between N-alkylaniline and β-(trifluoromethyl)-α,β-unsaturated enones was developed. The results of the control experiments show that the reaction may involve a radical mechanism by a single-electron transfer process. Moreover, a broad substrate scope and good functional groups, high diastereoselectivities (dr, up to >20 : 1) as well as gram-scale synthesis make this approach highly attractive.

 Please wait while we load your content...
Please wait while we load your content...