N-Heterocyclic carbene complexes enabling the α-arylation of carbonyl compounds†
Abstract
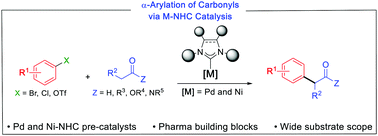
The considerable importance of α-arylated carbonyl compounds, which are widely used as final products or as key intermediates in the pharmaceutical industry, has prompted numerous research groups to develop efficient synthetic strategies for their preparation in recent decades. In this context, the α-arylation of carbonyl compounds catalyzed by transition-metal complexes have been particularly helpful in constructing this motif. As illustrated in this contribution, tremendous advances have taken place using palladium– and nickel–NHC (NHC = N-heterocyclic carbene) complexes as pre-catalysts for the arylation of a wide range of ketones, aldehydes, esters and amides with electron-rich, electron-neutral, electron-poor, and sterically hindered aryl halides or pseudo-halides. Despite significant progress, especially in asymmetric α-arylations promoted by chiral NHC ligands, there are numerous challenges which have and continue to encourage further studies on this topic. Some of these are presented in this report.
- This article is part of the themed collection: ChemComm Community – Dedicated Authors

 Please wait while we load your content...
Please wait while we load your content...