Pd-Catalyzed double carbopalladation/syn-insertion cascade reactions toward medium-size sulfoximine heterocycles†
Abstract
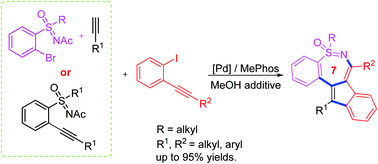
With the assistance of Ac in sulfoximine as a protecting group (PG) and MeOH as a de-PG agent, Pd-catalyzed multicomponent reactions were developed to access indene-fused medium-size sulfoximine heterocycles. The reactions proceeded smoothly under exceptionally mild conditions to produce polyheterocyclic sulfoximines with regiospecificity and good functional group tolerance. A double carbopalladation/syn-insertion of triple bond sequences was proposed tothis transformation.


 Please wait while we load your content...
Please wait while we load your content...