Conquering peaks and illuminating depths: developing stereocontrolled organic reactions to unlock nature's macrolide treasure trove
Abstract
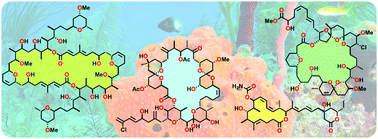
The structural complexity and biological importance of macrolide natural products has inspired the development of innovative strategies for their chemical synthesis. With their dense stereochemical content, high level of oxygenation and macrocyclic cores, we viewed the efficient total synthesis of these valuable compounds as an aspirational driver towards developing robust methods and strategies for their construction. Starting out from the initial development of our versatile asymmetric aldol methodology, this personal perspective reflects on an adventurous journey, with all its trials, tribulations and serendipitous discoveries, across the total synthesis, in our group, of a representative selection of six macrolide natural products of marine and terrestrial origin – swinholide A, spongistatin 1, spirastrellolide A, leiodermatolide, chivosazole F and actinoallolide A.


 Please wait while we load your content...
Please wait while we load your content...