α-Hydroxy boron-enabled regioselective access to bifunctional halo-boryl alicyclic ethers and α-halo borons†
Abstract
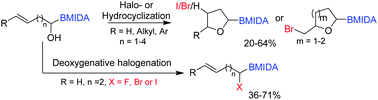
α-Hydroxy borons are an underutilized class of compounds and their only previous application involved oxidation into acylborons. Herein, we describe the synthesis of functionalized olefinic α-hydroxy borons and their utility to enable a novel and regioselective route to hitherto unknown bifunctional halo-boryl tetrahydrofurans/tetrahydropyrans and α-halo MIDA boronates. The orthogonally functionalized alicyclic ethers provided a building block-based approach for diversification of the tetrahydrofuran core.


 Please wait while we load your content...
Please wait while we load your content...