Zinc-catalyzed C–H alkenylation of quinoline N-oxides with ynones: a new strategy towards quinoline-enol scaffolds†
Abstract
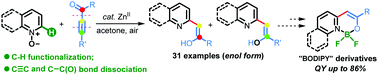
A zinc-catalyzed C–H alkenylation of quinoline N-oxides with ynones has been developed to rapidly assemble a broad collection of valuable quinoline-enol organic architectures. Uncommonly, this novel reaction involves C–H functionalization, and N–O, C–C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond cleavage in one operation, and leads exclusively to the formation of an enol rather than a keto product. Application of the enols generated was highlighted by further derivative transformation and preparation of a series of “BODIPY” analogues with high quantum yields (up to 86%).
C bond cleavage in one operation, and leads exclusively to the formation of an enol rather than a keto product. Application of the enols generated was highlighted by further derivative transformation and preparation of a series of “BODIPY” analogues with high quantum yields (up to 86%).


 Please wait while we load your content...
Please wait while we load your content...