Palladium-catalyzed decarbonylative and decarboxylative cross-coupling of acyl chlorides with potassium perfluorobenzoates affording unsymmetrical biaryls†
Abstract
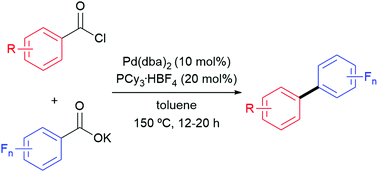
This paper describes the synthesis of unsymmetrical biaryls by the palladium-catalyzed cross-coupling reaction of acyl chlorides with potassium perfluorobenzoates. This transformation is unique in that it involves simultaneous decarbonylation and decarboxylation under redox-neutral conditions. Compared to conventional cross-coupling protocols for the synthesis of unsymmetrical biaryls, the two reactants in this synthetic strategy can be readily prepared from abundant and inexpensive aromatic carboxylic acids.


 Please wait while we load your content...
Please wait while we load your content...