Oxidative peptide bond formation of glycine–amino acid using 2-(aminomethyl)malononitrile as a glycine unit†
Abstract
Amide linkage of glycine–amino acid was synthesized by coupling of substituted 2-(aminomethyl)malononitrile as a C-terminal glycine unit and N-terminal amine using CsOAc and O2 in an aqueous solution. This is a coupling reagent-free and catalyst-free peptide synthesis via oxidative amide bond formation. Various tripeptides and tetrapeptides were synthesized efficiently and the sulfide moiety is inert even under an oxygen atmosphere.
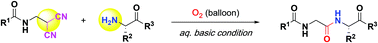


 Please wait while we load your content...
Please wait while we load your content...