Catalytic hydrogenation enabled by ligand-based storage of hydrogen†
Abstract
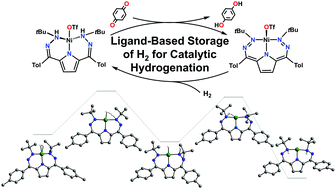
Biology employs exquisite control over proton, electron, H-atom, or H2 transfer. Similar control in synthetic systems has the potential to facilitate efficient and selective catalysis. Here we report a dihydrazonopyrrole Ni complex where an H2 equivalent can be stored on the ligand periphery without metal-based redox changes and can be leveraged for catalytic hydrogenations. Kinetic and computational analysis suggests ligand hydrogenation proceeds by H2 association followed by H–H scission. This complex is an unusual example where a synthetic system can mimic biology's ability to mediate H2 transfer via secondary coordination sphere-based processes.
- This article is part of the themed collections: Energy Frontiers: Hydrogen and Bioinspired metal complexes for chemical transformations and catalysis


 Please wait while we load your content...
Please wait while we load your content...