Carbene-mediated synthesis of a germanium tris(dithiolene)dianion†
Abstract
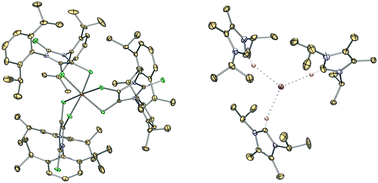
While the 1 : 1 reaction of 3 with an N-heterocyclic carbene ({(Me)CN(i-Pr)}2C:) in THF resulted in ligand-substituted product 4, the corresponding 1 : 2 reaction (in the presence of H2O) gives the first structurally characterized germanium tris(dithiolene)dianion 5 as the major product and the “naked” dithiolene radical 6˙ as a minor by-product. The structure and bonding of 4 and 5 were probed by experimental and theoretical methods. Our study suggests that carbene-mediated partial hydrolysis may represent a new method to access tris(dithiolene) complexes of main-group elements.


 Please wait while we load your content...
Please wait while we load your content...