Simple generation of various α-monofluoroalkyl radicals by organic photoredox catalysis: modular synthesis of β-monofluoroketones†
Abstract
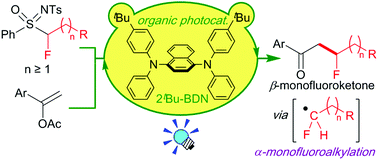
A metal-free and operationally simple strategy for the generation of various α-monofluoroalkyl radicals has been developed. A combination of 1,4-bis(diarylamino)naphthalene photocatalyst and sulfoximine-based fluoroalkylating reagents is the key to success. The protocol can be applied to modular synthesis of β-monofluoroketones through radical monofluoroalkylation of alkenyl acetates.


 Please wait while we load your content...
Please wait while we load your content...