Bifunctional amino sulfonamide-catalyzed asymmetric conjugate addition to alkenyl alkynyl ketimines as enone surrogates†
Abstract
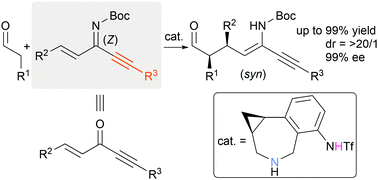
A bifunctional amino sulfonamide-catalyzed asymmetric conjugate addition of aldehydes to alkenyl alkynyl ketimines as reactive surrogates for enones has been developed. Use of a phenylcyclopropane-based amino sulfonamide catalyst, which can activate and orient the ketimines through hydrogen bonding, affords the desired conjugate adducts with high chemo-, diastereo- and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...