Complexation of 2,7-diazapyrene with boron for structural and electronic tuning†
Abstract
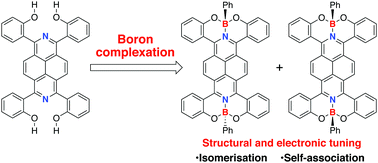
Complexation of 1,3,6,8-tetra(2-hydroxyphenyl)-2,7-diazapyrene with boron precursors provided the tetracoordinate diazapyrene boron complexes as separable anti- and syn-isomers. The structural difference of these isomers induces unique properties such as self-association behaviour of the syn-isomer and isomerisation of the anti-isomer in the solution and solid states.


 Please wait while we load your content...
Please wait while we load your content...