Depsipeptide synthesis using a late-stage Ag(i)-promoted macrolactonisation of peptide thioamides†
Abstract
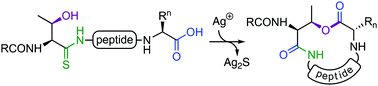
Macrolactonisation of peptides to generate cyclic depsipeptides is often challenging due to the low nucleophilicity of hydroxyl groups, epimerisation, cyclodimerisation, and potential acyl transfer reactions of the ester. Herein, we report a novel macrolactonisation strategy employing a Ag(I)-promoted conversion of peptide thioamides to isoimide intermediates, which undergo site-selective intramolecular acyl transfer to serine/threonine side chains to generate the macrolactone.


 Please wait while we load your content...
Please wait while we load your content...