Rapid synthesis of azepinoindole derivatives from tryptamine sulfonamides and bromoallyl sulfones via an acid-mediated cyclization and rearrangement†
Abstract
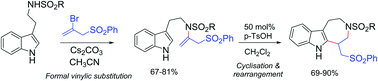
The azepinoindole framework present in natural alkaloids such as subincanadine F, ibogaine and catharanthine was formed in a novel acid-promoted cyclization–rearrangement of tryptamine-derived N-sulfonyl enamines. The latter were conveniently prepared via a cesium carbonate mediated formal vinylic substitution reaction of 2-bromoallyl sulfones (allenyl sulfone surrogate) and tryptamine sulfonamides. The azepinoindole forming cyclization–rearrangement involves the initial generation of a six-membered tetrahydro-β-carboline derivative. The steric bulk of the N-sulfonyl group on tryptamine plays an important role in deciding the reaction outcome.


 Please wait while we load your content...
Please wait while we load your content...