HFIP-catalyzed direct dehydroxydifluoroalkylation of benzylic and allylic alcohols with difluoroenoxysilanes†
Abstract
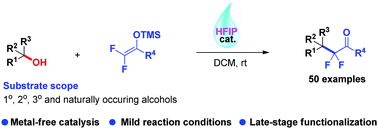
Hexafluoroisopropanol (HFIP)-catalyzed direct dehydroxydifluoroalkylation of benzylic and allylic alcohols with difluoroenoxysilanes is developed. This procedure enables the synthesis of a broad range of α,α-difluoroketones, a class of highly valuable intermediates and building blocks in medicinal and organic chemistry. Here, we have demonstrated for the first time that HFIP could act as a powerful catalyst for fluorinated carbon–carbon bond formation. The application of this protocol in late-stage dehydroxydifluoroalkylation of potentially bioactive drugs and natural products has also been carried out.


 Please wait while we load your content...
Please wait while we load your content...