Conjugated crosslinks boost the conductivity and stability of a single crystalline metal–organic framework†
Abstract
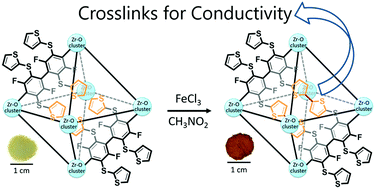
A linker molecule with four pendant thiophene functions was crystallized with Zr(IV) ions to form a semiconductive porous coordination solid (1.1 × 10−5 S cm−1). Oxidative treatment with FeCl3 guests then coupled the thiophene units to form conjugated bridges as covalent crosslinks. The resulting hybrid of a metal–organic framework and conjugated polymer featured robust crystalline order that withstood long-term air exposure and broad pH (from 0 to 12) conditions. Moreover, the homocoupled thiophene units, conjugated through sulfide links (–S–) with the linker backbone, afforded higher electronic conductivity (e.g., >2.2 × 10−3 S cm−1), which is characteristic of conductive polymer prototypes of polythiophene and polyphenylene sulfide. The crosslinked solid also exhibited proton conductivity that could be increased broadly upon H2SO4 treatment (e.g., from 5.0 × 10−7 to 1.6 × 10−3 S cm−1).


 Please wait while we load your content...
Please wait while we load your content...