Molecular-level insights into the self-assembly driven enantioselective recognition process†
Abstract
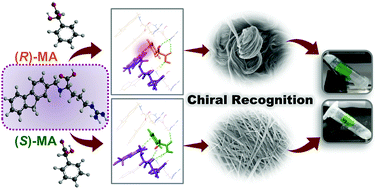
The importance of the orientation of functional groups in a chiral environment on enantioselective recognition has been demonstrated. Orientation controlled interactions of functional groups in (R)/(S)-MA lead to a visually differentiable morphology with an arginine-based gelator. The crucial role of various molecular–level interactions discriminating the enantioselective self-assembly has been established using different analytical techniques, crystal structure analysis, and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...