High-entropy alloys for water oxidation: a new class of electrocatalysts to look out for†
Abstract
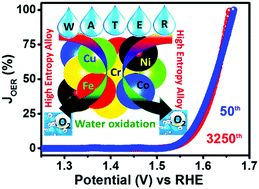
High-entropy alloys (HEAs) with five or more elements can provide near-continuous adsorption energies and can be optimized for superior persistent catalytic activity. This report presents electrochemical water oxidation facilitated by employing graphene and FeCoNiCuCr HEA nanoparticle based composites prepared via the mechanical milling of graphite-metal powders. The composite efficiently facilitates water oxidation with a low overpotential of 330 mV at 10 mA cm−2, and high specific and mass activities (∼143 mA cm−2 and 380 mA mg−1, respectively, at 1.75 V). Importantly, the composites exhibit excellent accelerated cycling stability with ∼99% current retention (after 3250 cycles). The HEA-based composites are anticipated to replace noble/precious metal based traditional electrocatalysts in the future, the use of which is a major obstacle in the technological scalability of electrochemical energy conversion and storage devices.


 Please wait while we load your content...
Please wait while we load your content...