A bicyclic S-adenosylmethionine regeneration system applicable with different nucleosides or nucleotides as cofactor building blocks†
Abstract
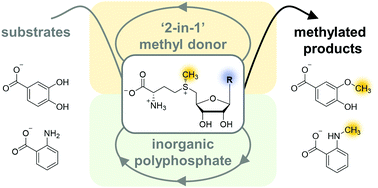
The ubiquitous cofactor S-adenosyl-L-methionine (SAM) is part of numerous biochemical reactions in metabolism, epigenetics, and cancer development. As methylation usually improves physiochemical properties of compounds relevant for pharmaceutical use, the sustainable use of SAM as a methyl donor in biotechnological applications is an important goal. SAM-dependent methyltransferases are consequently an emerging biocatalytic tool for environmentally friendly and selective alkylations. However, SAM shows undesirable characteristics such as degradation under mild conditions and its stoichiometric use is economically not reasonable. Here, we report an optimised biomimetic system for the regeneration of SAM and SAM analogues consisting of effective nucleoside triphosphate formation and an additional L-methionine regeneration cycle without by-product accumulation. The bicyclic system uses seven enzymes, S-methylmethionine as methyl donor and a surplus of inorganic polyphosphate, along with catalytic amounts of L-methionine and cofactor building block reaching conversions of up to 99% (up to 200 turnovers). We also show that the cycle can be run with cofactor building blocks containing different purine and pyrimidine nucleobases, which can be fed in at the nucleoside or nucleotide stage. These alternative cofactors are in turn converted to the corresponding SAM analogues, which are considered to be a key for the development of bioorthogonal systems. In addition to purified enzymes, the bicyclic system can also be used with crude lysates highlighting its broad biocatalytic applicability.


 Please wait while we load your content...
Please wait while we load your content...