A near-infrared fluorescence turn-on probe based on Michael addition–intramolecular cyclization for specific detection of cysteine and its applications in environmental water and milk samples and living cells†
Abstract
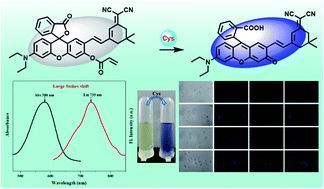
Owing to its important biological functions in many physiological and pathological processes, it is necessary to develop efficient and appropriate detection methods for monitoring the levels of Cys in biological systems. Based on this, a novel rhodol-isophorone derivative (RHI) was designed and synthesized as a reaction-based fluorescence probe for specific detection of Cys with high sensitivity and large Stokes shift (155 nm). This probe was composed of an acrylate moiety (recognition group) and a rhodol-isophorone derivative (fluorophore). Probe RHI could react with Cys rapidly (15 min) with a 100-fold fluorescence enhancement. The limit of detection value was calculated to be 0.168 μM. When Cys was added, the color of the probe RHI solution turned from yellow to blue, indicating that Cys could be monitored by the naked eye. In addition, probe RHI was successfully utilized for detecting Cys in environmental water and milk samples. More importantly, the probe could be applied to imaging Cys in living cells with low cytotoxicity and good biocompatibility.


 Please wait while we load your content...
Please wait while we load your content...