Using a 3-hydroxyflavone derivative as a fluorescent probe for the indirect determination of aminothiols separated by ion-pair HPLC†
Abstract
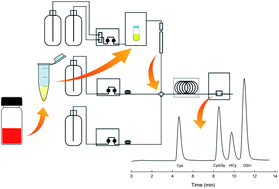
Homocysteine, cysteine, cysteinyl-glycine, and glutathione are significant biological aminothiols (ATs) that are marker-molecules in Down syndrome, Alzheimer's disease, or have been implicated as risk factors in atherosclerosis and other vascular diseases, and therefore rapid determination of these molecules is desirable. After reduction of the disulfides, a widely used method utilizes derivatization with ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate (SBD-F) as a fluorogenic probe prior to reversed-phase HPLC separation followed by fluorescence detection. The traditional HPLC determination of ATs is time consuming and economically expensive. We have developed an ion-pair HPLC method coupled with indirect fluorescence detection after post-column reaction with a 2,4-dinitrobenzenesulfonate derivative of a 3-hydroxyflavone. The accuracy, precision, post-column temperature and residence time, and limit-of-detection were evaluated. Sample throughput and reduced sample preparation time of over an hour for the existing methods to less than 20 minutes for the new method is also demonstrated. No statistical differences in HCy, Cys, or Cys-Gly determinations in plasma samples were observed between our method and the traditional HPLC method.


 Please wait while we load your content...
Please wait while we load your content...