A novel voltammetric method for the sensitive and selective determination of carbonate or bicarbonate ions by an azomethine-H probe†
Abstract
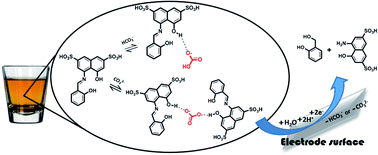
Our study involved a simple, sensitive voltammetric method of determining either carbonate or bicarbonate ions independently with azomethine-H and a disposable pencil graphite electrode. The reduction of azomethine-H–carbonate complexes at approximately −930 mV formed in acetic acid–acetate buffer solution (pH: 4.25) was evaluated as a response. Among the results, the limits of detection and analytical ranges for carbonate ions were 3.7 μg L−1 and 9.9–700.0 μg L−1 and for bicarbonate ions were 9.0 μg L−1 and 35.0–700.0 μg L−1, and the relative standard deviations for carbonate and bicarbonate ions ranged from 1.33% and 6.93% at different concentrations. After the proposed method was applied to water, sparkling water, seawater and baking powder samples, the results were statistically evaluated and compared with those obtained from the potentiometric auto-titration system. Last, the complex stoichiometry of both carbonate and bicarbonate ions was comprehensively investigated with fluorescence and 1H-NMR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...