Ultrasensitive electrochemical determination of phosphate in water by using hydrophilic TiO2 modified glassy carbon electrodes
Abstract
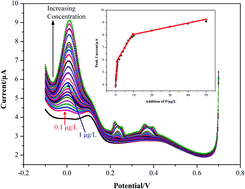
In this paper, ultrasensitive electrochemical determination of phosphate in water is achieved by using hydrophilic TiO2 modified glassy carbon electrodes for the first time. The differential pulse voltammetry (DPV) method is proposed to measure phosphate in water as pulse techniques offer higher sensitivity compared with the conventional cyclic voltammetry (CV) method. Hydrophilic TiO2 films were obtained upon ultraviolet (UV) illumination after TiO2 precursor emulsions were coated on the surfaces of glassy carbon electrodes, and used for phosphate determination. Contact angle measurements (around 23.4°) proved the good hydrophilicity of the TiO2 modified surface upon UV illumination. A detection limit of 0.1 μg L−1 is obtained, and a linear relationship (R2 = 0.99) between the phosphate concentration (ranging from 0.1 μg L−1 to 1 μg L−1) and the peak current was observed.


 Please wait while we load your content...
Please wait while we load your content...