Homochiral preference of serine octamer in solution and formed by dissociation of large gaseous clusters†
Abstract
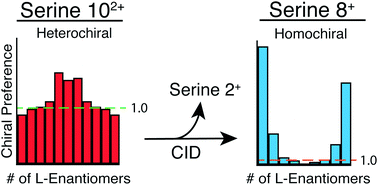
The ability of electrospray emitters with submicron tip diameters to significantly reduce and even eliminate aggregation of analyte molecules that can occur inside evaporating droplets was recently demonstrated to show that serine octamer exists in bulk solution, albeit in low abundance. Results using 222 nm emitter tips for D-serine and deuterium labeled L-serine show that the serine octamer that exists in 100 μM solution has a strong homochiral preference. Dissociation of large multiply protonated clusters results in formation of protonated octamer through a doubly protonated decamer intermediate. Remarkably, dissociation of the doubly protonated decamer from solution, which has a heterochiral preference, results in protonated octamer with strong homochiral preference. This homochiral preference is higher when protonated octamer is formed from larger clusters and approaches the chiral preference of the octamer in solution. These results show that the doubly protonated decamer has a different structure when formed from solution than when formed by dissociation of larger clusters. These results indicate that the unusually high abundance of protonated homochiral octamer formed by spray ionization methods that has been reported previously can be largely attributed to aggregation of serine that occurs in rapidly evaporating droplets and from dissociation of large clusters that form abundant protonated octamer at an optimized effective temperature.


 Please wait while we load your content...
Please wait while we load your content...
