Enhanced electrochemical biosensing on gold electrodes with a ferri/ferrocyanide redox couple
Abstract
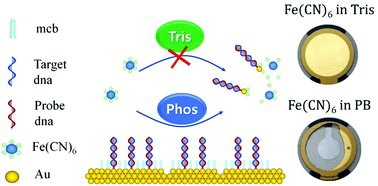
Detection of specific DNA is important in many fields. Label-free DNA sensing performed by electrochemical impedance spectroscopy (EIS) or using a quartz crystal microbalance (QCM) is widely employed for this purpose. Gold electrodes are mainly used for these techniques due to their chemical stability. However, ferro/ferricyanide used as a redox couple was found to etch the gold electrode and this significantly limited the repeatability of the EIS measurement. Inductively coupled plasma mass spectrometry (ICP-MS) and QCM experiments provided important clues about the gold dissolution mechanism and revealed that phosphate buffer promotes the dissolution of gold in the presence of the ferri/ferrocyanide redox couple. Tris buffered conditions, which provide the most stable environment, enabled the investigation of experimental parameters with a Q-sense electrochemistry module (QEM), which can perform QCM and EIS measurements simultaneously and revealed the principal factors that influence changes in the impedance. With the reproducible measurements, the estimation of an optimum probe-DNA concentration for detecting complementary DNA is demonstrated. In order to amplify the detection signal of target DNA, we sought to maximize the difference in response between the probe-only and target DNA by controlling the concentration of probe DNA. We showed that an intermediate probe-DNA concentration yields optimum signal amplification.


 Please wait while we load your content...
Please wait while we load your content...