Development of a piperazinyl-NBD-based fluorescent probe and its dual-channel detection for hydrogen sulfide†
Abstract
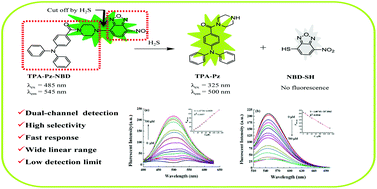
To selectively detect H2S based on the thiolysis reaction of 7-nitro-1,2,3-benzoxadiazole (NBD), amines attracted increasing attention since NBD amine is regarded as a new H2S reaction site. Herein, a novel fluorescent probe, triphenylamine piperazine NBD (TPA-Pz-NBD), was developed. The results showed that it exhibited high selectivity towards H2S via fluorescence spectroscopy and solution color. Furthermore, TPA-Pz-NBD not only detected H2S by a dual-channel, turn-on fluorescence signal at 500 nm and turn-off fluorescence signal at 545 nm, respectively, but also displayed a wide detection range of 0–125 μM. In addition, living cell imaging results indicated that TPA-Pz-NBD holds potential for the detection of intracellular H2S.
- This article is part of the themed collection: Analyst Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...