Near-infrared fluorescent organic porous crystal that responds to solvent vapors†
Abstract
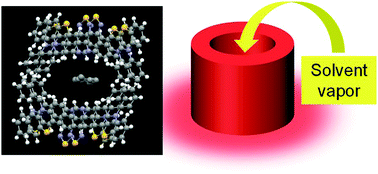
Organic porous fluorescent materials created by supramolecular noncovalent synthesis can be developed for fluorescent sensing applications. In this study, we report a noncovalent synthesis of a near-infrared porous fluorescent crystal and its solvent vapor-responsive fluorescence change. The donor–acceptor type phenothiazine–thiadiazolopyridine dye emits near-infrared fluorescence at 727 nm in the as-prepared state. The near-infrared emission band is shifted to 792 nm upon application of mechanical grinding and subsequent chloroform vapor-fuming. The resulting fumed sample has a porous crystal structure with a pore size larger than 5.75 Å. The electron-donating phenothiazine and electron-accepting thiadiazolopyridine moieties are closely one-dimensionally stacked through multiple noncovalent interactions, and the created one-dimensional architectures are two-dimensionally organized through an alternative arrangement of the phenyl groups on the phenothiazine rings, resulting in a three-dimensional porous structure. This near-infrared porous fluorescent crystal responds to the polarity difference of solvent vapors (from nonpolar hexane to polar methanol) incorporated inside the pore site, providing fluorescence wavelength and intensity changes.


 Please wait while we load your content...
Please wait while we load your content...