Zn(ii)/Cd(ii)-based metal–organic frameworks: crystal structures, Ln(iii)-functionalized luminescence and chemical sensing of dichloroaniline as a pesticide biomarker†
Abstract
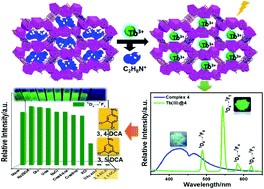
Mixing transition metal(II) salts with 3,5-di(2,4-dicarboxylphenyl)pyridine (H4pdda), 4,4′-bipyridine (4,4’-bpy) and 1H-tetrazole (HTz) led to the complexes [Zn2(pdda)H2O] (1), [Zn2(pdda)(bpy)0.5] (2), {[NH2(CH3)2]2·[Cd3(pdda)2(H2O)2]}n (3), and {[NH2(CH3)2]4·[Cd6(pdda)4(HTz)1.5(H2O)6]·3/4DMF·7/2H2O}n (4) ([NH2(CH3)2]+ = dimethylammonium cation, DMF = N,N′-dimethylformamide). These complexes were characterized by single-crystal X-ray diffraction. 1 possesses a dense 3D framework consisting of 2Zn dimer-linear chains and Zn2-pdda-Zn1-COO− helices involving {Zn1O3N} and {Zn2O5} polyhedra. 2 exhibits an open 3D framework with paddle-like binuclear Zn2(CO2)4 units, in which bpy as a brace bridges two units to support and segment the rhombus pores. 3 shows a grid-like 3D anionic framework constructed by pdda ligands and Cd3(μ2-OH2)2(CO2)4 units. 4 features a racemic 3D anionic framework with two kinds of chiral nanotube-like channels. The progressive structure variations from dense (1) to open frameworks (2, 3 and 4) are discussed systematically. These complexes exhibit different thermostabilities and chemical stabilities, and 4 shows single-crystal to single-crystal (SC–SC) phase transition in pure water. Moreover, Ln(III)-functionalized MOF hybrids, Ln(III)@4, are first fabricated by straightforward cation-exchange modification to replace [NH2(CH3)2]+ ions in channels of 4 with Ln(III) ions. These hybrids reveal notable and selective luminous sensitization of 4 to Tb(III) ions. Significantly, the Tb(III)@4 hybrid as a promising sensor has been proved for the first time to possess the potential of practical detection of biomarker dichloroanilines via multiple quenching mechanisms, and for the first time the detection of urinary dichloroanilines through fluorescence spectrometry based on a Ln-MOF sensor has been realized.


 Please wait while we load your content...
Please wait while we load your content...