Isomeric fused benzocarbazole as a chromophore for blue fluorescent organic light-emitting diodes†
Abstract
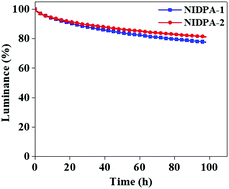
In the present work, we report two 7H-benzo[c]carbazole (BCz)-based isomeric chromophores, benzo[b]indolo[1,2,3-lm]carbazole (NI-1) and benzo[c]indolo[3,2,1-jk]carbazole (NI-2), formed by the fusion of BCz with a phenyl ring. Two blue fluorescent emitters, N6,N6,N12,N12-tetraphenylbenzo[b]indolo[1,2,3-lm]carbazole-6,12-diamine (NIDPA-1) and N10,N10,N13,N13-tetraphenylbenzo[c]indolo[3,2,1-jk]carbazole-10,13-diamine (NIDPA-2), were developed by functionalizing the NI-1 and NI-2 chromophores with diphenylamine donors, respectively. The influence of the fusion position on the light-emitting performances of the emitters was investigated. The NIDPA-1 emitter fused through the naphthalene unit of BCz showed a weak charge-transfer character, whereas the NIDPA-2 emitter fused through the phenyl unit of BCz displayed a strong charge-transfer character, which red-shifted the emission spectrum of NIDPA-2. The NIDPA-1 doped blue device showed a higher quantum efficiency and smaller full width at half maximum, and thus better blue color purity than the NIDPA-2 doped device. However, the NIDPA-2 device exhibited a longer device lifetime and maximum luminance (Lmax) above 70 000 cd m−2 compared to the NIDPA-1 device. However, the exceptional stability of both devices under high luminance (>60 000 cd m−2) and current density (>600 mA cm−2) demonstrates the significance of the NI-based chromophores in developing stable blue emitters for OLED applications.


 Please wait while we load your content...
Please wait while we load your content...