Molecular anchoring stabilizes low valence Ni(i)TPP on copper against thermally induced chemical changes†
Abstract
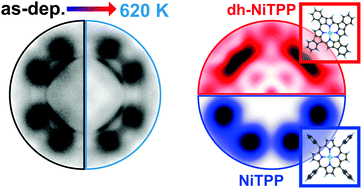
Many applications of molecular layers deposited on metal surfaces, ranging from single-atom catalysis to on-surface magnetochemistry and biosensing, rely on the use of thermal cycles to regenerate the pristine properties of the system. Thus, understanding the microscopic origin behind the thermal stability of organic/metal interfaces is fundamental for engineering reliable organic-based devices. Here, we study nickel porphyrin molecules on a copper surface as an archetypal system containing a metal center whose oxidation state can be controlled through the interaction with the metal substrate. We demonstrate that the strong molecule–surface interaction, followed by charge transfer at the interface, plays a fundamental role in the thermal stability of the layer by rigidly anchoring the porphyrin to the substrate. Upon thermal treatment, the molecules undergo an irreversible transition at 420 K, which is associated with an increase of the charge transfer from the substrate, mostly localized on the phenyl substituents, and a downward tilting of the latters without any chemical modification.


 Please wait while we load your content...
Please wait while we load your content...