Neutral pH aqueous redox flow batteries using an anthraquinone-ferrocyanide redox couple
Abstract
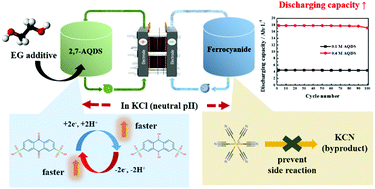
Anthraquinone-2,7-disulfonic acid (2,7-AQDS) and ferrocyanide including potassium and sodium salts are used as a redox couple for neutral aqueous redox flow batteries (ARFBs). In 1 M potassium chloride (KCl) electrolyte of neutral pH, the electron transfer rate and redox reactivity of 2,7-AQDS are better than those in acidic electrolyte. Furthermore, ferrocyanide is attacked by the hydroxyl group within the alkaline electrolyte, however this does not happen in KCl because there are no hydroxyl groups in KCl. The low solubility of 2,7-AQDS is enhanced in 1 M KCl using an ethyleneglycol (EG) additive and the solubility of the ferrocyanide also increases when using the mixed cations of potassium and sodium. This benefit arises because of the fact that the EG additive forms hydrogen bonds with water within the electrolyte and 2,7-AQDS, while ferrocyanide mixed with different cations increases the solubility due to ion pair formation. Based on that, the ARFB using 2,7-AQDS and ferrocyanide shows high coulombic efficiency (98% at 40 mA cm−2) and capacity (17.8 A h L−1) with a low capacity decay rate (>99% until 100 cycle).


 Please wait while we load your content...
Please wait while we load your content...