Growth dynamics and photoresponse of the Wadsley phase V6O13 crystals†
Abstract
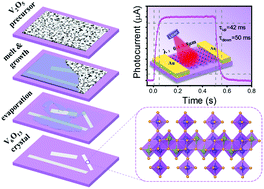
The preparation of a pure phase has long been the key obstacle for the fundamental research and device application of Wadsley vanadium oxides (VnO2n+1) due to the mixed-valence feature and closeness in thermodynamic phase diagrams. Herein, we demonstrate a melt-assisted pyrolysis process to prepare pure V6O13 (n = 6) using a V2O5 precursor film. V6O13 with an atomic flat (00l) terrace and length up to a millimeter was prepared on a c-cut sapphire surface. Both ex situ and in situ real-time investigations on the growth process reveal that the melting and decomposition of V2O5 started as synchronization processes for the nucleation of V6O13. The endothermic melting process provides the main driving force for the rapid growth of V6O13 crystals along the melt/solid interface. The as-prepared V6O13 crystal sheets show a broadband photoresponse capability (0.4–8.8 μm) with a rise/fall time of 42 ms/50 ms, and the maximum EQE of 5.4 × 104%. Spatial photocurrent imaging reveals that both photoelectric and bolometer effects contribute to the photoresponse. This study offers a feasible and scalable method for the preparation of high quality mix-valence vanadium oxide for future opto-electrical and energy storage devices.


 Please wait while we load your content...
Please wait while we load your content...