The optical signatures of molecular-doping induced polarons in poly(3-hexylthiophene-2,5-diyl): individual polymer chains versus aggregates†
Abstract
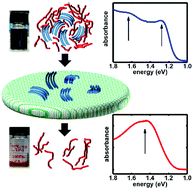
Optical absorption spectroscopy is a key method to investigate doped conjugated polymers and to characterize the doping-induced charge carriers, i.e., polarons. For prototypical poly(3-hexylthiophene-2,5-diyl) (P3HT), the absorption intensity of molecular dopant induced polarons is widely used to estimate the carrier density and the doping efficiency, i.e., the number of polarons formed per dopant molecule. However, the dependence of the polaron-related absorption features on the structure of doped P3HT, being either aggregates or separated individual chains, is not comprehensively understood in contrast to the optical absorption features of neutral P3HT. In this work, we unambiguously differentiate the optical signatures of polarons on individual P3HT chains and aggregates in solution, notably the latter exhibiting the same shape as aggregates in solid thin films. This is enabled by employing tris(pentafluorophenyl)borane (BCF) as dopant, as this dopant forms only ion pairs with P3HT and no charge transfer complexes, and BCF and its anion have no absorption in the spectral region of P3HT polarons. Polarons on individual chains exhibit absorption peaks at 1.5 eV and 0.6 eV, whereas in aggregates the high-energy peak is split into a doublet 1.3 eV and 1.65 eV, and the low-energy peak is shifted below 0.5 eV. The dependence of the fraction of solvated individual chains versus aggregates on absolute solution concentration, dopant concentration, and temperature is elucidated, and we find that aggregates predominate in solution under commonly used processing conditions. Aggregates in BCF-doped P3HT solution can be effectively removed upon simple filtering. From varying the filter pore size (down to 200 nm) and thin film morphology characterization with scanning force microscopy we reveal the aggregates' size dependence on solution absolute concentration and dopant concentration. Furthermore, X-ray photoelectron spectroscopy shows that the dopant loading in aggregates is higher than for individual P3HT chains. The results of this study help understanding the impact of solution pre-aggregation on thin film properties of molecularly doped P3HT, and highlight the importance of considering such aggregation for other doped conjugated polymers in general.


 Please wait while we load your content...
Please wait while we load your content...