Obvious ferroelectricity in undoped HfO2 films by chemical solution deposition
Abstract
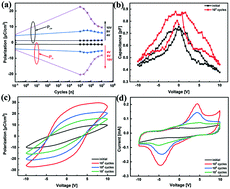
Although great achievements have been made in realizing ferroelectricity in HfO2-based films by the ALD method, the performance is strongly constrained by film thickness and dopant types. This study was the first to realize ferroelectric properties in undoped HfO2 films by the chemical solution deposition method, which broke the above restrictions while using the ALD method. The evolution of ferroelectricity in pure HfO2 films was studied over a wide range of thickness from 34 nm to 136 nm, without doping any other metallic elements. The HfO2 film with a thickness of 136 nm exhibited a large remnant polarization (Pr) of 22.56 μC cm−2 after a wake-up process of 105 cycles, and could endure up to 107 switching cycles. Residual carbon from the incomplete decomposition of organic matter in films and growth disruption of grains through layer-by-layer thermal treatment could lead to the reduction in grain size, which was beneficial to the formation of the metastable ferroelectric o-phase. Oxygen vacancies existing at the interfaces between the TiN bottom electrode and HfO2 film could be rearranged during electric field cycling, thus inducing the appearance of ferroelectricity. The movement of domains under different tip bias by a piezo force microscope verified the existence of ferroelectricity in the undoped HfO2 films. This report therefore provides an inexpensive and feasible way to gain ferroelectricity in pure HfO2 films and paves the way toward other sensor applications beyond thickness limitations and doping systems.


 Please wait while we load your content...
Please wait while we load your content...