High catalytic efficiency from Er3+-doped CeO2−x nanoprobes for in vivo acute oxidative damage and inflammation therapy†
Abstract
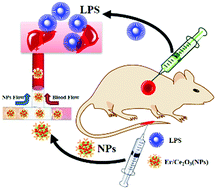
Cerium oxide nanoparticles (NPs) due to their advanced catalytic performance have been widely used to treat oxidative damage. However, Ce2O3 NPs have not been further investigated in the treatment of acute oxidative injury in vivo. It is meaningful to improve the efficiency for treatment of acute oxidative injury with NPs in vivo. In this report, we designed Er3+-doped Ce2O3 (Er/Ce2O3) NPs with a size of 7.9 nm, which were used to treat acute liver injury. Er/Ce2O3 NPs realized high-efficiency catalysis of hydrogen peroxide (H2O2) at room temperature. An acute liver damage model was established through intraperitoneal injection of lipopolysaccharide (LPS) in C57 mice. By analyzing histopathological and biochemical indexes, Er/Ce2O3 NPs showed a significant improvement in LPS-induced acute liver injury. Acute liver oxidative damage can be treated within 24 hours, which proved the high catalytic efficiency of Er/Ce2O3 NPs in vivo. The activities of SOD, GPx and CTA increased and production of ROS decreased with Er/Ce2O3 NP treatment in comparison with LPS-induced injury, indicating that the mechanism of Er/Ce2O3 NPs in the treatment of acute oxidative damage of liver was mainly via catalysis of ROS products. Moreover, the protein expression levels of TNF-α, CD45 and IL-1β in liver decreased in the Er/Ce2O3 NPs-treated group, which indicated that Er/Ce2O3 NPs have the function of anti-inflammation property. Therefore, Er/Ce2O3 NPs can be applied to treat and prevent diseases caused by acute oxidative damage.


 Please wait while we load your content...
Please wait while we load your content...