pH-triggered solubility and cytotoxicity changes of malachite green derivatives incorporated in liposomes for killing cancer cells†
Abstract
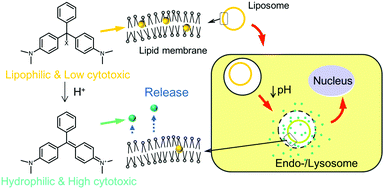
Three different malachite green leuco derivatives (MG-Xs) are incorporated in liposomes. In all three cases, a substituent (X) is covalently linked to the central carbon atom, abbreviated as MG-OH, MG-OCH3, and MG-CN. The three MG-X compounds are solubilized separately in liposome membranes and become cationic (MG+) and water soluble under acidic conditions. MG+ is consequently released from the liposome to the aqueous exterior. Their release behavior corresponds to their ionization ability: MG-OH > MG-OCH3 > MG-CN. The cellular uptake of the liposomes, the cytotoxic effect, and the location of MG+ in cancer cells are investigated using murine cells derived from colon cancer (Colon 26 cells) and human embryonic kidney cells (HEK 293 cells). The toxic effect on cancer cells is correlated to the ionization ability of MG-Xs. The liposomes effectively deliver MG+via the endocytic pathway, resulting in the cytotoxicity of liposomes containing MG-OH which is higher than that of free MG-OH and MG+. The difference in the phospholipids constituting the liposome membranes barely had an effect on the ionization ratio and the cytotoxicity of MG-OH. Confocal fluorescence microscopic observations revealed that MG+ is ultimately transported into the nuclei after being released in acidic cellular compartments.


 Please wait while we load your content...
Please wait while we load your content...