Enzyme-responsive turn-on nanoprobes for in situ fluorescence imaging and localized photothermal treatment of multidrug-resistant bacterial infections†
Abstract
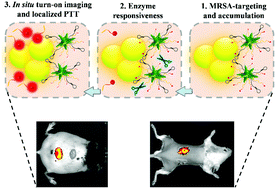
Sensitive diagnosis and elimination of multidrug-resistant bacterial infections at an early stage remain paramount challenges. Herein, we present a gelatinase-responsive turn-on nanoprobe for in situ near-infrared (NIR) fluorescence imaging and localized photothermal treatment (PTT) of in vivo methicillin-resistant Staphylococcus aureus (MRSA) infections. The designed nanoprobe (named AuNS–Apt–Cy) is based on gold nanostars functionalized with MRSA-identifiable aptamer and gelatinase-responsive heptapeptide linker (CPLGVRG)–cypate complexes. The AuNS–Apt–Cy nanoprobe is non-fluorescent in aqueous environments due to the fluorescence resonance energy transfer between the gold nanostar core and cypate dye. We demonstrate that the AuNS–Apt–Cy nanoprobe can achieve MRSA targeting and accumulation as well as gelatinase (overexpressed in MRSA environments)-responsive turn-on NIR fluorescence due to the cleavage of the CPLGVRG linker and localized in vitro PTT via a mechanism involving bacterial cell wall and membrane disruption. In vivo experiments show that the AuNS–Apt–Cy nanoprobe can enable rapid (1 h post-administration) and in situ turn-on NIR fluorescence imaging with high sensitivity (105 colony-forming units) in diabetic wound and implanted bone plate mouse models. Remarkably, the AuNS–Apt–Cy nanoprobe can afford efficient localized PTT of diabetic wound and implanted bone plate-associated MRSA infections under the guidance of turn-on NIR fluorescence imaging, showing robust capability for early diagnosis and treatment of in vivo MRSA infections. In addition, the nanoprobe exhibits negligible damage to surrounding healthy tissues during PTT due to its targeted accumulation in the MRSA-infected site, guaranteeing its excellent in vivo biocompatibility and solving the main bottlenecks that hinder the clinical application of PTT-based antibacterial strategies.


 Please wait while we load your content...
Please wait while we load your content...