A AuNP-capped cage fluorescent biosensor based on controlled-release and cyclic enzymatic amplification for ultrasensitive detection of ATP†
Abstract
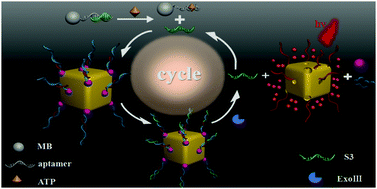
Gold nanodevices have attracted extensive interest in the detection of specific targets within cells. However, constructing gold sensing devices that can be activated by the simulation of remote applications remains a huge challenge. Here, we report a Au nanoparticle (AuNP)-capped cage fluorescent biosensor based on controlled-release and Exonuclease III (Exo III) assisted cyclic enzymatic amplification that can be activated by adenosine triphosphate (ATP). In the system, AuNPs were used as the building blocks to cap the pores of Au nanocages (AuNCs) loaded with Rhodamine B (RhB) molecules through the hybridization of DNA. The RhB fluorescent molecules were finally released with the help of Exo III in the presence of ATP for detection purposes. Ultimately, the biosensor leads to a wide linear ATP detection range from 1.0 × 10−9 to 1.0 × 10−7 M with a limit of detection (LOD) down to 0.88 nM. In addition, it also has good selectivity for ATP to distinguish between ATP and ATP analogues such as cytidine triphosphate (CTP), guanosine triphosphate (GTP), and uridine triphosphate (UTP). Therefore, as a convenient and sensitive biosensor, it is expected to be widely used in the biomedical field.


 Please wait while we load your content...
Please wait while we load your content...