Gold nanoparticle-mediated generation of reactive oxygen species during plasmonic photothermal therapy: a comparative study for different particle sizes, shapes, and surface conjugations†
Abstract
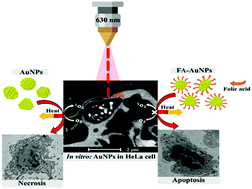
Gold nanoparticle (AuNP)-mediated photothermal therapy represents an alternative to the effective ablation of cancer cells. However, the photothermal response of AuNPs must be tailored to improve the therapeutic efficacy of plasmonic photothermal therapy (PPT) and mitigate its side effects. This study presents an alternative to ease the tuning of photothermal efficiency and target selectivity. We use laser-treated spherical and anisotropic AuNPs of different sizes and biocompatible folic acid (FA)-conjugated AuNPs (FA–AuNPs) in the well-known human epithelial cervical cancer (HeLa) cell line. We show that large AuNPs produce a more significant photothermal heating effect than small ones. The thermal response of the spherical AuNPs of 9 nm was found to reach a maximum increase of 3.0 ± 1 °C, whereas with the spherical AuNPs of 14 nm, the temperature increased by over 4.4 ± 1 °C. The anisotropic AuNPs of 15 nm reached a maximum of 4.0 ± 1 °C, whereas the anisotropic AuNPs of 20 nm reached a significant increase of 5.3 ± 1 °C in the cell culture medium (MEM). Notably, the anisotropic AuNPs of 20 nm successfully demonstrate the potential for use as a photothermal agent by showing reduced viability down to 60% at a concentration of 100 μM. Besides, we reveal that high concentrations of reactive oxygen species (ROS) are formed within the irradiated cells. In combination with stress by photothermal heating, it is likely to result in significant cell death through acute necrosis by compromising the plasma membrane integrity. Cell death and ROS overproduction during PPT were characterized and quantified by transmission electron microscopy (TEM) and confocal fluorescence microscopy with different fluorescent markers. In addition, we show that FA–AuNPs induce cell death through apoptosis by internal damage, whereas diminish the ROS formation during PPT treatment. Our findings suggest the ability of plasmon-mediated ROS to sensitize cancer cells and make them vulnerable to photothermal damage, as well as the protective role of FA–AuNPs from excessive ROS formation, whereas reducing the risk of undesired side effects due to the necrotic death pathway. It allows an improvement in the efficacy of the AuNP-based photothermal therapy and a reduction in the number of exposures to high temperatures required to induce thermal stress.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...