Nanoclay-functionalized 3D nanofibrous scaffolds promote bone regeneration†
Abstract
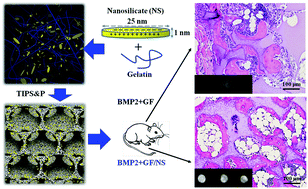
Developing a biomaterial that can promote osteoblastic differentiation, thereby reducing the needs of exogenous osteogenic factors for large bone repair, has been a significant and long-term technical hurdle. In this study, we developed an innovative nanoclay (nanosilicate, NS)-functionalized 3D gelatin nanofibrous scaffold (GF/NS) through a thermally induced phase separation method together with the particle leaching technique (TIPS&P). In addition to the significantly higher mechanical strength, the composite scaffolds (GF/NS) demonstrated a significantly stronger ability to promote the osteogenic differentiation of human mesenchymal stem cells (hMSCs) in vitro compared to the GF scaffold. Our data further revealed that this intriguing pro-osteoblastic functionality was largely because of the unique features of NS, particularly, the strong binding ability to pro-osteoblastic factors (e.g., BMP2) as well as the intrinsic osteoinductivity of its bioactive degradation products. Most importantly, our in vivo studies indicated that GF/NS scaffolds significantly improved low-dose BMP2-induced ectopic bone regeneration in mice.


 Please wait while we load your content...
Please wait while we load your content...