High pressure chemical reactivity and structural study of the Na–P and Li–P systems
Abstract
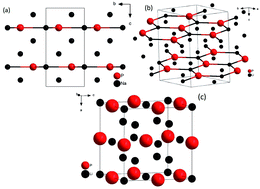
The Na–P and Li–P chemical systems were studied under pressure using synchrotron X-ray diffraction in a diamond anvil cell up to 20 GPa, combined with the AIRSS ab initio random structure searching technique. The results reveal an enhanced reactivity of both alkali metals with phosphorous at slightly elevated pressures. This enables the synthesis of Li3P and Na3P at room temperature (RT) starting from element precursors, bypassing the established chemical synthesis methods. Both compounds undergo a pressure-induced phase transition from the hexagonal Na3As-type structure (stable at ambient conditions) towards a Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (FCC) structure that remains stable up to 20 GPa. Attempts to synthesize compounds with higher alkali metal content (such as Li5P) using high-temperature and -pressure conditions (up to 2000+ K and 30 GPa), inspired by recent theoretical predictions, were not successful.
m (FCC) structure that remains stable up to 20 GPa. Attempts to synthesize compounds with higher alkali metal content (such as Li5P) using high-temperature and -pressure conditions (up to 2000+ K and 30 GPa), inspired by recent theoretical predictions, were not successful.


 Please wait while we load your content...
Please wait while we load your content...