Realizing electronic modulation on Mo sites for efficient hydrogen evolution reaction†
Abstract
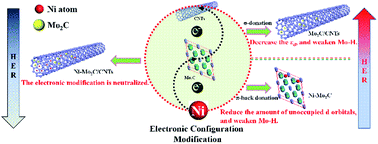
Electronic modulation is of great significance for electrocatalysts, as it can intrinsically improve their catalytic properties. Here we realize the electronic strong metal–support interaction (ESMSI) and the electron injection effect on Mo sites by introducing carbon nanotube (CNT) supports and Ni-doping. Compared with the pristine molybdenum carbide (Mo2C), the sufficient variation in electron density around Mo (either decreased or increased) can enhance the activity for catalyzing the hydrogen evolution reaction (HER) by optimizing the Mo–H bond strength, where the former leads to reduced electrons in the bonding orbitals and the latter results in electron transfer into the anti-bonding orbitals. However, a hybrid electrocatalyst, synthesized by combining the two strategies together, shows a decrease in the HER properties due to the neutralizing effect. The discussion about electronic modulation in this work may open up new opportunities to develop high-performance catalysts via rational manipulation of the electronic structure.


 Please wait while we load your content...
Please wait while we load your content...