Destabilization of LiBH4 by the infusion of Bi2X3 (X = S, Se, Te): an in situ TEM investigation†
Abstract
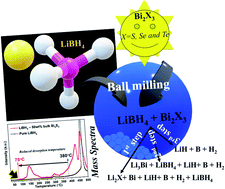
Lithium borohydride is a potential candidate not only for hydrogen storage (high hydrogen capacity ∼18.5 wt%), but it can also be used as a solid electrolyte due to the high conductivity (∼10−3 S cm−1) of its high-temperature phase. However, it cannot be utilized as a hydrogen storage material practically because of its high stability and high decomposition temperature. During our recent work on LiBH4 as a solid electrolyte for Bi2X3 anode materials in all-solid-state Li-ion batteries, a thermochemical side reaction was observed. This thermochemical reaction restricted the potential window of electrochemical cycling, thus lowering the overall capacity of the electrode material. However, on the other hand, it allowed a significant destabilization of LiBH4. This motivated us to investigate the effect of bismuth chalcogenides on the stability of LiBH4 in detail. In this work, we prepared LiBH4 composites with bulk and nanostructures of Bi2X3 and investigated the minimum decomposition temperature of LiBH4. In order to understand the detailed reaction mechanism, X-ray diffraction (XRD) and Transmission Electron Microscopy (TEM) were performed after milling as well as at different temperatures according to TG-DTA experiments. The onset desorption temperature of LiBH4 could be lowered down to 50 °C by the infusion of 50 wt% Bi2S3 nanoflowers. This work not only enabled us to achieve efficient destabilization of LiBH4 but also provided a significant understanding of the side reaction during the use of LiBH4 as a solid electrolyte for Bi2X3 chalcogenides. It will be helpful in tuning the electrolyte–electrode combination without any side reaction, thus improving the cycling stability.


 Please wait while we load your content...
Please wait while we load your content...