Oxygen defect engineering in double perovskite oxides for effective water oxidation†
Abstract
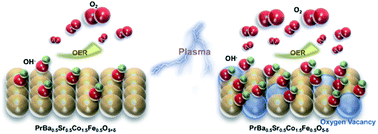
Owing to the sluggish reaction kinetics of the oxygen evolution reaction (OER), the development of high-efficiency OER electrocatalysts is of great importance for many energy devices. Oxygen defect engineering has been reported as an effective approach to regulate the OER activity of transition metal oxides. Generating oxygen defects in a controllable manner, however, remains a challenging task, and the impact of oxygen defects on the OER kinetics still requires further investigation. Herein, taking the PrBa0.5Sr0.5Co1.5Fe0.5O5+δ (PBSCF) perovskite thin film as a model material, we generate oxygen vacancies with various concentrations in the material by Ar and H2 plasma treatment. Electrochemical tests showed that the OER activity of PBSCF was significantly boosted with addition of oxygen vacancies. In addition, the pH dependence test showed that oxygen defect formation in PBSCF leads to decoupled proton and electron transfer in the OER process. The surface adsorption characteristics modified by oxygen defects are believed to be the reason for enhanced performance and modified reaction kinetics. In addition, we applied plasma processing to the powder material. At a current density of 35 mA cm−2, the overpotential of the obtained defective PBSCF powder is 90 mV lower than that of commercialized IrO2 catalysts, suggesting its good OER performance at high current density. The methodology used in this work can be generally applied to understanding and controlling anionic defects in other oxide, sulphide and phosphide catalysts for a variety of energy and environmental applications.


 Please wait while we load your content...
Please wait while we load your content...