The conversion reaction mechanism of bimetallic Ni–Fe hydroxycarbonate and its encapsulation in carbon nanospheres for achieving excellent Li-ion storage performance†
Abstract
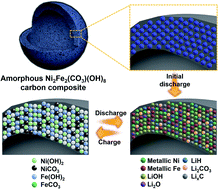
The search for promising anode materials with optimum compositions for use in lithium ion batteries (LIBs) is still underway. Herein, the lithium storage mechanism of nickel iron hydroxycarbonate (NiFeHC) consisting of two transition metal cations and two anion compounds is investigated. Through various analysis tools and in situ and ex situ techniques, it was found that the following reversible conversion reactions occur from the second cycle onward: (1) M(OH)2 + MCO3 + 4Li+ + 4e− ↔ 2M + Li2CO3 + 2LiOH (M = Ni, Fe), (2) Li2CO3 + (4 + 0.5x)Li+ + (4 + 0.5x)e− ↔ 3Li2O + 0.5LixC2 (x = 0, 1, 2), and (3) LiOH + 2Li+ + 2e− ↔ Li2O + LiH. It is known that heterostructured composite materials with different band gaps exhibit attractive electrochemical performance owing to the presence of a heterointerface that forms an intrinsic internal electric field. When tested as an anode, the crystalline NiFeHC material exhibited a high reversible capacity of 1221 mA h g−1 in the initial cycle when cycled at 1 A g−1. To enhance the electrochemical properties of the NiFeHC material, amorphous NiFeHC was formed within the shells of hollow carbon nanospheres via in situ precipitation, yielding a-NiFeHC@C. a-NiFeHC@C exhibited stable cycle performance up to 700 cycles and high rate performance, where a discharge capacity of 251 mA h g−1 was achieved at a high current density of 30 A g−1. The synergy of the heterointerface and encapsulation of amorphous NiFeHC within the carbon shell enabled excellent electrochemical properties.


 Please wait while we load your content...
Please wait while we load your content...