Elucidating the optical, electronic, and photoelectrochemical properties of p-type copper vanadate (p-Cu5V2O10) photocathodes†
Abstract
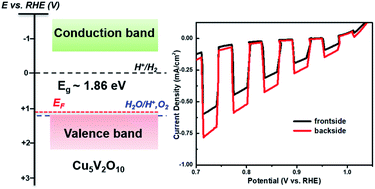
P-type copper vanadate (Cu5V2O10) photoelectrodes made by spray pyrolysis were developed and evaluated as a potential photocathode material for photoelectrochemical (PEC) water splitting. Using fluorine-doped tin oxide as a substrate, highly phase-pure p-Cu5V2O10 thin films were obtained after annealing at 550 °C for 4 hours in air. Cu5V2O10 has a small bandgap energy in the range of 1.8–2.0 eV, allowing it to absorb visible light and making it potentially interesting for solar water splitting applications. The p-Cu5V2O10 films were characterized by photoelectrochemical techniques in order to provide insight into the critical PEC properties such as the flat-band potential, chemical stability, and incident photon-to-current efficiency (IPCE). The best-performing films showed a photocurrent density of up to 0.5 mA cm−2 under AM1.5 simulated sunlight, and an IPCE value up to 14% for 450 nm light at 0.8 VRHE with H2O2 as an electron scavenger. Despite the narrow band gap and suitable conduction band edge position for PEC H2 production, these p-type films were unstable under constant illumination in aqueous electrolyte (pH 6.8) due to the reduction and dissolution of Cu. Based on our findings, the suitability of Cu5V2O10 as a photocathode material for photoelectrochemical water splitting is critically discussed.


 Please wait while we load your content...
Please wait while we load your content...