Engineering the electronic structure of 1T′-ReS2 through nitrogen implantation for enhanced alkaline hydrogen evolution†
Abstract
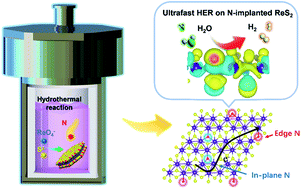
The electrocatalytic hydrogen evolution reaction (HER) in water–alkali and chlor–alkali electrolyzers is an indispensable source of H2 supply for industrial applications. Transition metal dichalcogenides (TMDs) have been demonstrated to be highly promising for the HER, but they are mostly operated in acidic solutions. Rationally designing TMD electrocatalysts towards efficient alkaline HER still remains challenging. Herein, we report a controllable atomic engineering of nitrogen atoms into 1T′-phase ReS2 using a simple one-step method. The optimized material shows a greatly enhanced HER performance under alkaline conditions, achieving a low overpotential of 90 mV at 10 mA cm−2 and outstanding stability (with negligible activity decay during the 140 h test). Density functional theory calculations reveal that rational N-implanting remarkably promotes H2O dissociation and H adsorption/desorption on the edge active sites of ReS2, and meanwhile increases electronic states near the Fermi level for improved charge transfer. Such ensemble effects are proposed to account for the much improved HER activity. The work broadens the horizon for designing TMD materials via atomic-site engineering for alkaline HER or other catalysis reactions.


 Please wait while we load your content...
Please wait while we load your content...