A catalyst design for selective electrochemical reactions: direct production of hydrogen peroxide in advanced electrochemical oxidation†
Abstract
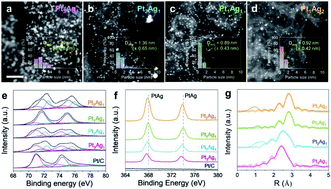
Hydrogen peroxide production by enhanced electrocatalysts is an attractive alternative to the present commercial process. While the subnano/atomic dispersion in noble metal nanocatalysts is known to strongly enhance their catalytic efficiency and chemoselectivity, their excessive surface energy and consequent coarsening seriously compromise their physical/chemical stability. Here, we report a subnano/atomically dispersed Pt–Ag alloy (by a simply modified polyol process) that is resistant to agglomeration/Ostwald ripening. This catalyst does not follow a conventional four-electron oxygen reduction reaction (ORR) but selectively produces H2O2 without excessive degradation of its activity. We clarified the role of the alloying element, Ag, as follows: (1) selective activation of two-electron ORR by inhibiting O2 dissociation and (2) suppression of H2O2 decomposition by preventing the H2O2 adsorption. The present approach provides a convenient route for the direct generation of H2O2 as a simple byproduct of electricity generation by fuel-cell systems.


 Please wait while we load your content...
Please wait while we load your content...