Enhanced electrocatalytic activity and selectivity of glycerol oxidation triggered by nanoalloyed silver–gold nanocages directly grown on gas diffusion electrodes†
Abstract
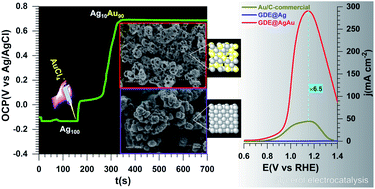
There is currently great interest for the development of electrochemical reactors to be implemented for the emerging world-class reactions of hydrogen evolution (HER), CO2 reduction (CO2RR) and N2 reduction (N2RR) in order to electro-synthesize high value-added chemicals. However, precisely how this could be achieved in a cheap and sustainable way remains a puzzle for scientists since the electrochemical kinetics of the oxygen evolution reaction (OER) is the major limitation. Increasing interest surrounds the development of low energy consumption electrolyzers capable of oxidizing selectively biomass-based organics instead of water for a dual production of chemicals (paired electrosynthesis). Also, the state-of-the-art electrocatalyst is in the form of nanoparticles either dispersed on carbon supports or in liquids in the presence of stabilizers to prevent the nanoparticles' aggregation, detachment and performance loss. In this report, free-standing surfactant- and binder-free silver–gold nanoporous and nanoalloyed catalysts are successfully synthesized via a simple and effective approach directly on a gas diffusion electrode (GDE@AgAu). Physicochemical and electrocatalytic analyses show that the obtained activity and selectivity of metallic nanocatalysts towards the glycerol electrooxidation reaction in alkaline media depend on their morphology and composition. Specifically, an onset potential of Eonset = 0.3 V vs. RHE and a record peak current density of jpeak = 18 A mgAu−1 (290 mA cm−2) at GDE@AgAu in comparison with those of the tested commercial Au/C catalyst (Eonset = 0.7 V vs. RHE and jpeak = 2.8 A mgAu−1 or 44 mA cm−2) were obtained. Also, an unprecedented selectivity in glycerol electrooxidation towards two products of high interest, formate (66%) and glycolate (32%), has been obtained. GDE@AgAu is thus a potential substitute as an anode material for commercial Au and Pt-based catalysts for efficient alcohol electroreforming with low energy consumption and cogeneration of H2, CO2 or N2 electrolyzers. The present method that combines direct growth, galvanic replacement and nanostructuration provides a smart route for creating new designs for catalyst nanofabrication in order to target high electrocatalytic performance.


 Please wait while we load your content...
Please wait while we load your content...